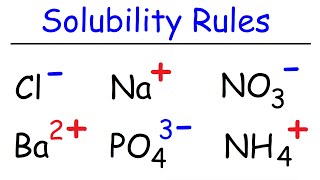
Solubility Rules: Explanation & Practice
The solubility rules are important for quickly figuring out is a substance is soluble or insoluble in water. Often students are required to memorize the rules for solubility shown below:
-----
Top rules supersede any lower rules.
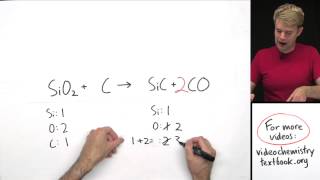
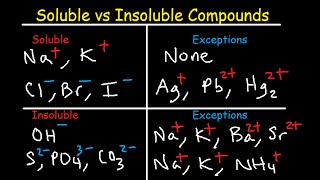
In general, salts of:
* Group I elements (Li+, Na+, K+, Cs+, Rb+) are soluble.
* NH4+ are soluble.
* the nitrate ion (NO3-) are generally soluble.
* of Cl-, Br-, and I- are soluble. Exceptions Ag+, Pb2+, and (Hg2)2+
* most sulfates are soluble. Exceptions: Ba2+, Ca2+, Pb2+, Ag+, Sr2+ .
*most hydroxide salts are only slightly soluble. Exceptions: NH4+, Li+, Na+, K+
---
In this video we’ll introduce and practice these rules, building your memory as we go. Then we add several other rules that you’ll see from time to time.
Check with your instructor to see which solubility rules you need to memorize. Some teachers allow you to use a solubility chart but you may still want to have the most common rules memorized.
Note that all compounds are soluble to some degree. If that is a very small amount, we say they are insoluble.
Also note, that when we discuss solubility, remember that solubility is affected by temperature, generally becoming more soluble as the temperature increases.














![Intro to Chemistry & What is Chemistry? - [1-1-1]](https://ytimg.googleusercontent.com/vi/pdyDmXtye2w/mqdefault.jpg)

